Table of Contents
ToggleMushroom Solutions, a trailblazer in innovative healthcare and life sciences solutions, proudly announces the launch of its Clinical Trials Operations Automation Solutions (CTOps). This state-of-the-art platform is poised to transform the landscape of clinical research by addressing critical challenges in participant management and document processing
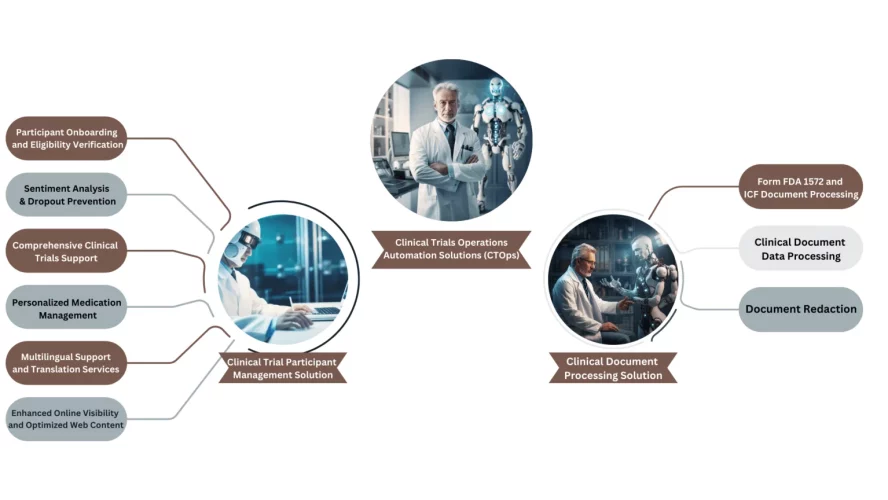
Clinical Trials Reimagined: Mushroom Solutions’ CTOps is a holistic and advanced suite of solutions designed to streamline and elevate every facet of clinical trials. From seamless participant onboarding and eligibility verification to comprehensive clinical trials support, personalized medication management, and multilingual services, CTOps ensures an unparalleled level of efficiency and accuracy in trial operations
Key Features:
Participant Onboarding and Eligibility Verification: Seamlessly integrate participants into clinical trials with our robust onboarding system. Conduct thorough eligibility checks to ensure only qualified individuals are enrolled, enhancing trial integrity and efficiency.
Sentiment Analysis and Dropout Prevention: Gauge participant feedback and sentiment with our advanced sentiment analysis tools. Employ proactive strategies to prevent participant dropout and enhance engagement throughout the trial duration.
Comprehensive Clinical Trials Support: From site selection and patient recruitment to data management and regulatory compliance, Mushroom Solutions provides comprehensive support and guidance. Ensure the smooth and successful execution of clinical trials with our expert assistance.
Personalized Medication Management: Benefit from our personalized medication management support, including dosage tracking and adherence monitoring. Promote participant safety and optimize treatment outcomes with our tailored assistance.
Multilingual Support and Translation Services: Bridge language barriers with our multilingual support and translation services. Ensure clear communication and understanding among diverse participant populations, enhancing inclusivity and accessibility.
Enhanced Online Visibility: Elevate the online visibility of clinical trial services with our strategic search engine optimization (SEO) techniques. Increase exposure and attract potential participants to relevant trial opportunities with our tailored approach.
Optimized Web Content: Maximize the effectiveness of your web content, including websites and digital platforms, with our web content optimization services. Deliver a user-friendly experience that encourages participation and promotes the advancement of medical research.
Benefits of Clinical Trials Operations Automation Solutions (CTOps):
Streamlined Enrolment Process:
CTops ensures a seamless and efficient participant onboarding process, reducing administrative burden and accelerating trial initiation.
Streamlined processes contribute to quicker enrolment, allowing researchers to focus more on the core aspects of the trial.
Enhanced Trial Integrity:
Thorough eligibility checks are conducted to ensure only qualified participants are enrolled, maintaining the integrity of the trial and enhancing the reliability of collected data.
Rigorous verification processes contribute to data accuracy, minimizing the risk of errors in trial outcomes.
Improved Participant Engagement:
Utilizing advanced sentiment analysis tools, CTOps gauges participant feedback and sentiment, allowing for proactive strategies to prevent dropout and enhance overall engagement.
Improved participant engagement leads to higher retention rates, ensuring a more comprehensive and representative study population.
Comprehensive Support:
Mushroom Solutions provides end-to-end support, from site selection to regulatory compliance, ensuring a smooth and successful execution of clinical trials.
Comprehensive support minimizes potential bottlenecks in the trial process and enhances overall operational efficiency.
Personalized Medication Management:
CTOps includes personalized medication management support, offering dosage tracking and adherence monitoring for participants.
This feature enhances participant safety, ensures adherence to treatment protocols, and contributes to optimized treatment outcomes.
Inclusive Communication:
Multilingual support and translation services bridge language barriers, fostering clear communication and understanding among diverse participant populations.
Inclusive communication ensures that trials are accessible to a broader range of participants, contributing to the diversity and representativeness of the study cohort.
Increased Trial Visibility:
Elevating online visibility through strategic Search Engine Optimization (SEO) techniques attracts more potential participants to relevant trial opportunities.
Increased trial visibility enhances recruitment efforts, ensuring a robust participant pool for research studies.
Optimized Web Content:
CTOps helps maximize the effectiveness of web content, including websites and digital platforms, providing a user-friendly experience that encourages participation.
Optimized web content contributes to a positive participant experience, driving increased interest and engagement in clinical trials.
Enhanced Data Quality:
Personalized medication management and rigorous eligibility verification contribute to maintaining high-quality data throughout the trial.
High data quality ensures reliable trial outcomes, supporting the credibility of research findings.
Accelerated Time-to-Market:
CTOps expedites the entire trial process, from participant recruitment to data analysis, accelerating the development and commercialization of new treatments.
Faster time-to-market is crucial for bringing innovative therapies to patients more quickly.
Improved Patient Experience:
Providing participants with a seamless and user-friendly experience enhances satisfaction and willingness to participate in future trials.
Improved patient experiences contribute to positive perceptions of clinical research and promote continued participation.
Competitive Edge:
Differentiate trials from competitors by offering advanced participant management capabilities, attracting more sponsors, collaborators, and stakeholders.
The competitive edge gained through CTOps positions research initiatives as leaders in the evolving landscape of clinical trials.
In summary, CTOps from Mushroom Solutions is a comprehensive solution that not only addresses operational challenges but also brings tangible benefits to the efficiency, effectiveness, and success of clinical trials.