CMC Automation
One solution for eCTD: Module 3 & Annual Reports
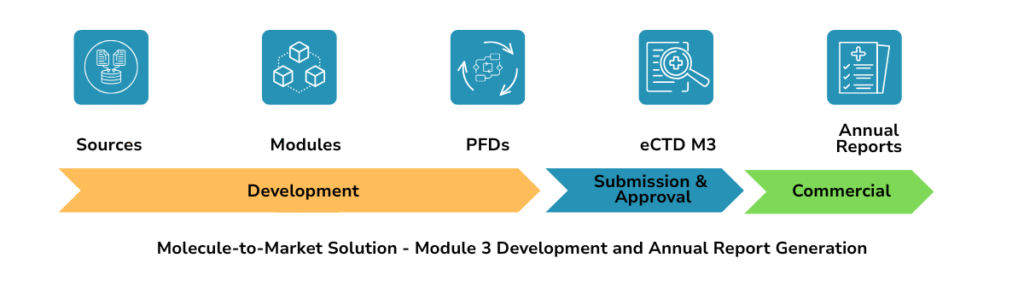
Bridging Fragmented Workflows
Teams lose time collating data across QMS/LIMS/SharePoint, re-entering values, and reconciling versions. Mushroom automates extraction → structuring → authoring with full traceability—so your eCTD stays consistent from development to commercial lifecycle updates.
Solution Benefits
Compliance
Authoring aligned to FDA/EMA eCTD formats and organization templates (e.g., ICH M11).
Consistency
Annual Reports reuse the approved Module 3 as a single source of truth with current-year updates.
Speed
Auto-compile drafts from validated data & prior filings.
Traceability
Link every value back to origin with audit trails
Scalability
Multi-site, multi-modality, DS/DP version control.
Lifecycle: Development and Commercial
Development eCTD (Module 3)
- Ingest & normalize assay/PPQ/release/stability/risk
- Map to CMC modules (3.2.S / 3.2.P) with templates
- PFDs linked to specs/IPC & facility fit
- Version matrix: DS/DP by site (e.g., Site I DS V1 / DP V2)
- Generate Module 3 drafts → eCTD sequence

Benefits

Interactive PFDs

PPQ & Release

Module 3 Authoring

Commercial eCTD (Annual Reports)
- Reuse approved Module 3 + current-year data.
- Auto-summaries: stability trends, deviations/CAPA, spec/site/version changes.
- Change history & RFI roll-ups.
- Export report packages → eCTD sequence.
Benefits

Annual Reports Automation

Smart Summaries
Export & Traceability
Real-World Use Case
Business Challenge
A pharmaceutical manufacturing company faced recurring delays and inefficiencies in CMC submissions. Over 4,000 manual hours were spent annually across 100+ products due to fragmented data, manual compilation, and inconsistent templates.

- Automated Data Extraction & Structuring: Integrated QMS and LIMS to auto-ingest and standardize critical CMC data.
- Dynamic Module 3 Generation: Enabled one-click eCTD draft creation using predefined templates for all modalities.
- Change Tracking & Version Control: Automated document update detection with streamlined review workflows.
- System Integration: Connected Regulatory, Quality, and Manufacturing teams through Salesforce and Document Center integration.
- Time Savings: Reduced CMC preparation time.
- Enhanced Accuracy: Minimized manual errors, ensuring data consistency across all modules.
- Regulatory Readiness: Delivered audit-ready, submission-compliant documents for global agencies.
- Scalability: Supported automation for all products and modalities, improving visibility and cross-functional collaboration.
Ready to see your data flow into eCTD?
Bring sample documents—we’ll show ingestion → modules/PFDs → Module 3 & Annual Reports.




















Enter details to access demo
Please enter your details before accessing demo
Our Solutions Gallery

CONTACT US
Please feel free to reach out
Ready to enhance your Pharmacovigilance operations? Reach out to Mushroom Solutions to learn more about our tailored solutions for the life sciences industry. Our team of experts is here to assist you in ensuring the safety and efficacy of your pharmaceutical products
























