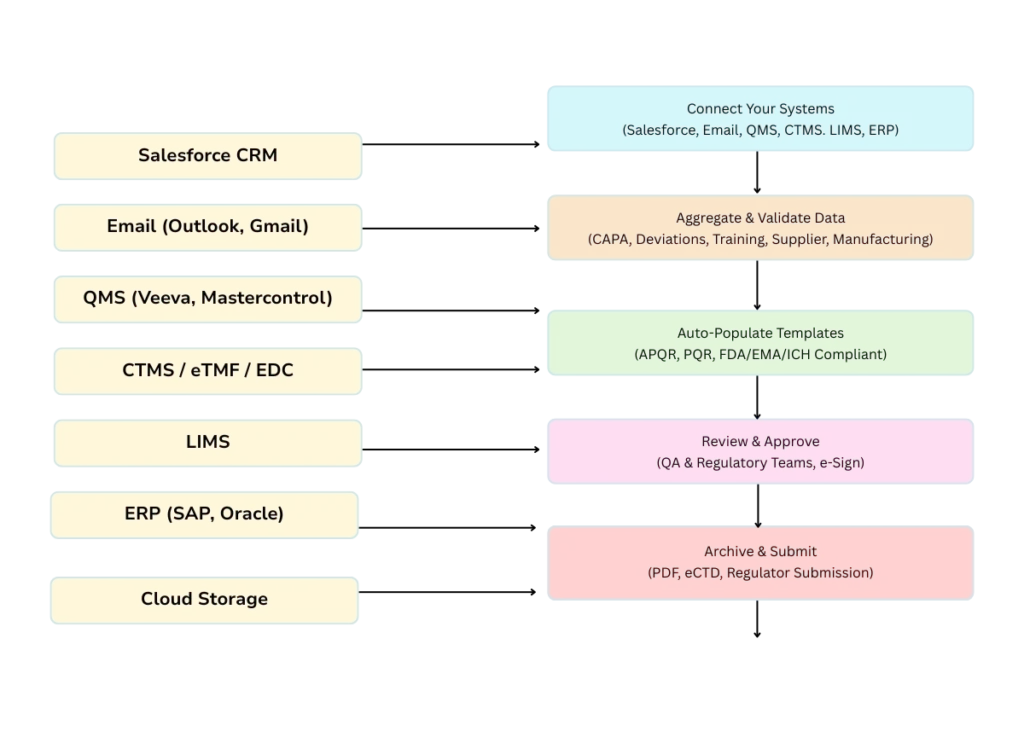
QMS Integration
Manually compiling Annual Product Quality Reviews (APQRs) or Periodic Quality Reviews (PQRs) eats up weeks, risks errors, and strains your quality teams. Our QMS integration framework automates data collection from all your systems—Salesforce, Email, LIMS, ERP, eTMF, and more—into regulator-ready reports. Maintain audit readiness, save time, and ensure 100% data accuracy.

Solution Features
Salesforce CRM Integration
Pull sponsor communications, site interactions, and trial milestones into quality reports.
Email System Integration
Automatically extract quality-relevant communications, deviation notices, and CAPA (Corrective and Preventive Actions) approvals.
QMS Platforms (Track Wise)
Directly fetch audit logs, change control records, training completion status, and deviations.
Clinical Trial Systems
Integrate and extract data from various clinical trial systems: CTMS for operational metrics eTMF for document completeness checks EDC for data quality metrics LIMS for lab results and stability studies
ERP Systems (SAP, Oracle ERP)
Integrate manufacturing batch data, supplier performance, and material quality records.
Cloud Storage & Collaboration Tools
Link supporting evidence directly into the report package.
Regulatory Submission Tools
Sync with eCTD or regulatory publishing platforms for faster submissions.
How It Works
Benefits
Time Savings
Reduce report preparation time by 70%+
Regulatory Compliance
Built to align with FDA, EMA, and ICH Q10
Increased Accuracy
Pull only validated, up-to-date data
Audit Readiness
Maintain digital trails for every report step
Boost Collaboration
Enable cross-functional teams to work on the same document in real time
Enter Details To Access White Papper




















Enter details to access demo
Please enter your details before accessing demo
Our Solutions Gallery




CONTACT US
Please feel free to reach out
Embrace our Participant Onboarding & Engagement solution to transform your clinical trials with seamless, secure, and engaging participant experiences.
























