Optimizing Clinical Trial Budgeting: How AI-Powered CTOps by Mushroom Solutions Eliminates Hidden Costs and Accelerates Timelines
Clinical research sites often encounter numerous financial challenges due to hidden costs that aren’t covered by standard study budgets. These additional expenses, ranging from administrative overheads to unforeseen regulatory requirements, can put a strain on research sites and hinder the progress of clinical trials. Addressing these hidden costs through fair and equitable budgeting is essential for the long-term sustainability of clinical research.
Mushroom Solutions’ CTOps, a comprehensive Clinical Trials Operations Automation Solution, offers an effective approach to managing these challenges. By leveraging AI and automation technologies, CTOps empowers Sponsors, CROs, and research sites to streamline budgeting processes, enhance financial transparency, and ensure compliance. The result is a reduction in costs and an acceleration of trial timelines.

The Hidden Costs in Clinical Trials
Traditional clinical trial budgets often miss key expenditures that arise during the lifecycle of a study. These hidden costs can lead to budget overruns, delayed timelines, and financial strain on research sites. Common hidden costs include:
- Administrative Overheads: Costs related to study coordination, documentation, and regulatory compliance. Ethics and regulatory approvals can take 6–12 months, delaying trial initiation and increasing overhead
- Unforeseen Regulatory Requirements: Additional expenses due to audits, compliance checks, or evolving regulations.
- Participant Recruitment and Retention: Expenses related to recruiting patients, retaining participants, and compensating them. Recruitment accounts for 30–40% of trial budgets, with delays driving overall costs up by 25%.
- Data Management and Monitoring: The cost of data collection, analysis, and monitoring throughout the study. Phase III trials now generate 283% more data points than a decade ago, stretching resources and extending timelines
These unaccounted-for expenses can cause delays, budget mismanagement, and strain the resources of research sites.

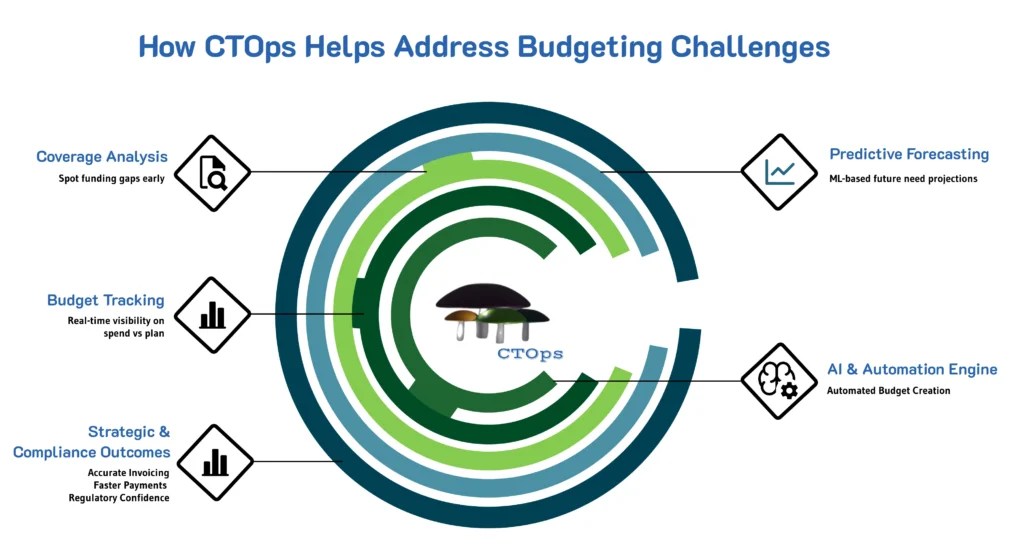
How CTOps Helps Address Budgeting Challenges
Mushroom Solutions’ CTOps integrates AI and automation to help overcome the financial challenges faced by clinical research sites:
1. Automated Budget Creation and Tracking
CTOps automates the creation of detailed budgets by analyzing historical data and forecasting future expenses. This reduces manual errors, ensures comprehensive cost coverage, and provides real-time tracking of expenditures against the allocated budget.
2. Dynamic Coverage Analysis
The platform offers real-time coverage analysis, identifying gaps in funding and ensuring that all study activities are covered. This proactive approach helps prevent financial shortfalls, allowing for timely budget adjustments.
3. Predictive Budgeting
By using machine learning algorithms, CTOps predicts future financial needs based on ongoing trial data and historical trends. This foresight helps Sponsors and CROs allocate resources effectively, minimizing the risk of budget overruns.
4. Automated Invoice Processing and Payment Management
CTOps automates the invoicing process by generating invoices, approving workflows, and processing payments. This reduces administrative burden, speeds up payment cycles, and ensures financial accuracy.
5. Regulatory Compliance and Reporting
CTOps ensures compliance with regulatory standards by automating compliance checks and generating necessary reports. This reduces the risk of compliance-related delays and the associated costs.

Benefits for Sponsors, CROs, and Research Sites
By adopting CTOps, clinical trial stakeholders can experience several key benefits:
- Cost Savings: Prevents overruns by reducing hidden costs. Eliminating unnecessary procedures and using adaptive design can save $500K–$800K per trial.
- Accelerated Timelines: Automation and predictive workflows can cut administrative delays, reducing trial timelines by up to 30–50%.
- Enhanced Financial Transparency: Real-time expense tracking provides clear visibility into financial status, improving decision-making.
- Improved Compliance: Automated compliance checks minimize costly audit findings and mitigates the risk of delays in multi-jurisdictional trials.
- Optimized Resource Allocation: Predictive analytics for budgeting ensures that resources are allocated efficiently throughout the study.
Real-World Impact and Use Cases
CTOps has demonstrated its capabilities in improving efficiency, ensuring audit-ready coverage compliance, and real-time insights for better budget control:
Efficiency Gains & Time Savings
Sites and CROs can reallocate resources from manual reconciliation to higher‑value tasks like patient engagement and trial optimization.
90% reduction in payment admin hours; payments now under 35 days vs 75 days prior
Consistent, Audit‑Ready Coverage Compliance
Ensures financial clarity in coverage decisions and smoother audit outcomes for sponsors and research sites.
50% faster report prep; 20% cost reduction; FDA-compliant output
Real‑Time Insights & Cost Control
Enables better budget adherence and financial decision‑making during ongoing trials, improving compliance and reducing unnecessary costs.
Live dashboards for site/data performance, better budget control, fewer overages

Conclusion
Mushroom Solutions’ CTOps provides a comprehensive solution to hidden costs in clinical trial budgeting through AI-powered automation. By offering tools for automated budget creation, dynamic coverage analysis, predictive budgeting, and streamlined compliance, CTOps enables Sponsors, CROs, and research sites to manage trials more efficiently, reduce costs, and accelerate timelines.
As the clinical trial landscape continues to evolve, adopting automation and AI-driven solutions like CTOps is essential to ensuring that trials are both scientifically rigorous and financially sustainable.


