Is your regulatory team ready for the next FDA Deficiency Letters?
FDA’s Center for Devices and Radiological Health (CDRH) reports over 55% of 510(k) submissions receive at least one Additional Information or Deficiency Letter—often due to missing data, unclear testing, or labeling issues.
Across the product lifecycle, from preclinical to post-approval, life sciences companies face intense regulatory scrutiny, evolving standards, and increasingly tight timelines. FDA Deficiency Letters, Clinical Holds, and Refuse to File (RTF) decisions have become all-too-common roadblocks.
This blog explores the types of FDA Deficiency Letters, their impact on review cycles, and how bespoke regulatory compliance automation and AI solutions —like Mushroom Solutions’ FDA Search Bot—help streamline response management and accelerate review cycles.
What Are FDA Deficiency Letters?
An FDA Deficiency Letter is a formal communication from the FDA indicating that your submission contains deficiencies that must be addressed before approval or further review can proceed.
Types of Deficiency Letters include:
Information Request (IR) Letters
Sent during the review process to clarify or request additional data.
Additional Information or Deficiency Letters (For Medical Devices – 510(k), PMA)
Sometimes the FDA may request further clarification, testing, or data to address identified issues before a medical device can be cleared or approved.
Complete Response Letters (CRLs)
Issued when the FDA has completed reviewing a New Drug Application (NDA), Biologics License Application (BLA), or Premarket Approval (PMA) but cannot approve it in its current form. This is considered one of the most critical types of review letters that detail deficiencies, missing data, or required actions for potential future approval.
Refuse to File (RTF) or (Pre-approval submissions)
Sent when the FDA deems an application (typically NDA, BLA) incomplete or insufficient for full review. This FDA Review Letter is sent often due to missing data, inadequate study design, or major documentation gap in a New Drug Application (NDA) or Biologics License Application (BLA).
Clinical Hold Letters (IND Applications)
Issued during Investigational New Drug (IND) application reviews if the FDA identifies significant safety concerns or deficiencies that must be addressed before clinical trials can proceed.
These letters often highlight gaps in areas like:
- Safety or efficacy data
- Manufacturing processes
- Labeling or instructions for use
- Clinical trial design
The High Stakes of FDA Deficiency Letters
Regulatory submissions are rigorous by design across the life sciences industry.
Companies can face:
- Delays due to Deficiency Letters or Complete Response Letters (CRLs)
- Costly rework from incomplete or incorrect submissions
- Market entry setbacks impacting patient access and revenue
- Incomplete documentation triggering Refuse to File (RTF) decisions
Unfortunately, traditional regulatory teams often rely on:
- Manual tracking of FDA deficiency letters and review letters
- Disconnected databases with limited search functionality
- Slow, error-prone response drafting
- No centralized regulatory management system
- Limited visibility into historical trends
So how can life sciences teams reduce delays and respond to FDA Deficiency Letters faster?
Streamlining FDA Deficiency Letters Review Cycle with Automation and AI
The shift toward automated regulatory reporting and regulatory intelligence software is transforming how companies manage compliance.
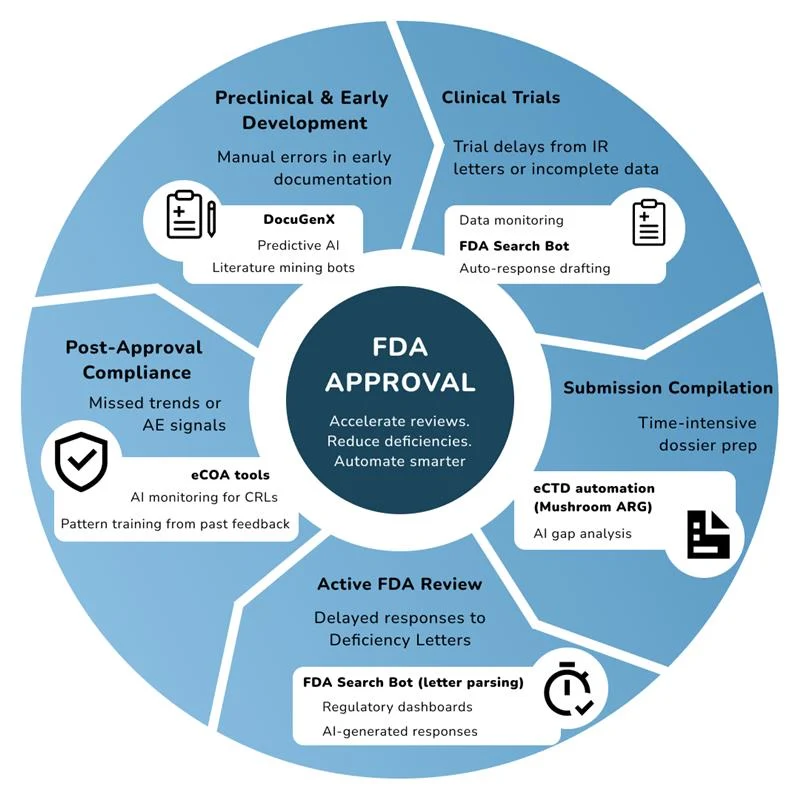
1. Preclinical & Early Development
Automated Document Preparation solutions like DocuGenX can extract, standardize, and pre-fill regulatory documents, reducing manual errors and time.
Predictive Regulatory Intelligence AI models can analyze thousands of historical FDA Deficiency Letters, Clinical Hold decisions, and approval trends to flag potential gaps before submission.
Intelligent Literature & Data Mining AI searches scientific literature, public FDA databases, and precedent decisions to support robust IND/IDE submissions.
These early-stage solutions fall under regulatory automation, providing strategic insights and reducing rework.
2. Clinical Trial Stage
During trials, real-time tracking of deficiencies and automated response generation and updating of regulatory documentation is critical in reducing trial time.
- AI-driven platforms monitor clinical data for missing, erroneous, or non-compliant entries, reducing the risk of trial disruptions.
- AI-Powered Data Monitoring & Validation Real-time trial data checks to ensure integrity, completeness, and regulatory compliance.
- Automated Response Drafting to IR/DR Letters AI-generated first drafts for responses to FDA Information Requests, saving time on regulatory writing.
- FDA Search Bot facilitates deficiency trend analysis on previous FDA communications to guide protocol adjustments and risk mitigation during trials.
- Together, these solutions embody automated regulatory intelligence and support regulatory compliance automation in life sciences.
3. Submission Preparation
AI accelerates this phase by automating dossier assembly, performing AI-powered gap analysis, and ensuring eCTD compliance.
AI-Assisted Submission Compilation Automated assembly of technical dossiers, converting diverse formats into FDA-compliant structures (eCTD, etc.)
Automated Gap Analysis AI reviews submission packages to identify missing data or sections that typically trigger Deficiency or RTF Letters
Document Translation & Formatting Automation Speeds up preparation of global regulatory documents, reducing human error
Tools like:
- Mushroom’s Annual Reports Generation for automated eCTD submission preparation
- Generis CARA for AI-driven document quality checks
- Language AI solutions for accurate translations and formatting
4. Active Review Period
FDA reviews often include IR Letters, Deficiency Letters, or mid-cycle feedback. Responding swiftly and accurately is crucial.
- FDA Search Bot facilitates precise searches for new IR, DR, or Deficiency Letters
- AI-Supported Rapid Response Generative AI drafts scientific, clinical, and manufacturing responses based on past successful submissions and regulatory language patterns
- Regulatory Analytics Dashboards Visualize submission progress, track pending requests, and assess response quality using AI-driven dashboards
These solutions represent an advanced level of regulatory reporting automation, ensuring agility during the most sensitive phase.
5. Post-Approval & Continuous Learning
Even after approval, AI plays a vital role in ensuring ongoing compliance. For example, Mushroom Solutions’ eCOA assists through adverse event (AE) monitoring and reporting.
Plus, by training AI models on historical FDA feedback, companies continuously refine their submission strategies, reducing risk in future applications.
- AI Analysis of Complete Response Letters (CRLs) Automated extraction of deficiencies, suggested corrective actions, and risk forecasting for resubmission
- Compliance Automation for Post-Market Surveillance AI monitors adverse event data, real-world evidence, and ensures ongoing regulatory compliance
- Training AI Models with Historical Deficiency Patterns Improves future submission quality by continuously learning from past feedback
- With these tools, companies address both regulatory risk and legal compliance automation post-approval, strengthening long-term readiness.
The Competitive Advantage of Intelligent Regulatory Processes
Companies embracing AI-powered regulatory compliance automation enjoy:
- Faster submission cycles
- Reduced risk of deficiencies and delays
- Cost savings through process efficiency
- Improved regulatory success rates
- Greater focus for teams on innovation, not paperwork
Conclusion: The Future of FDA Submissions is Digital
In an industry where time-to-market and compliance are mission-critical, AI and automation aren’t optional — they’re becoming essential.
By embedding regulatory intelligence software and automation tools across the submission lifecycle, life sciences organizations can
modernize outdated workflows, reduce time-to-market, and boost their chances of approval.


