Smarter Clinical Trials with AI in SDTM Automation
50% of biopharmaceutical professionals identified either the variety of data types (26%) or lack of standardization (24%) as their primary challenges when dealing with external data sources in clinical trials. In clinical research, data consistency is the key to seamless operations and high data quality maintenance across multiple systems, teams, and trial sites involved. This leads to the pressing need for a standardized structure for data which Study Data Tabulation Model (SDTM) stands for.
Developed by the Clinical Data Interchange Standards Consortium (CDISC), SDTM provides a standardized format for organizing data collected during clinical trials. Regulatory authorities like the FDA (U.S.) and PMDA (Japan) require clinical data to be submitted in SDTM format for review and approval.
The Challenges of Manual SDTM Mapping
While SDTM ensures consistency and regulatory readiness, the process of converting raw clinical data into SDTM format is far from simple. Clinical data is not available or collected in SDTM-ready form—it needs to be carefully transformed. Traditionally, this involves:
- Integration of data from disparate sources
- Manual mapping of collected data to SDTM domains
- Custom specification creation for each study
- Time-consuming data cleaning and formatting
- A high risk of human error and inconsistencies
It takes, on average, 6-8 weeks to manually map and generate SDTM making it difficult to manage and analyze large amounts of data typically generated during clinical trials. Manual SDTM generation processes delay timelines, increase costs, and can lead to rework if submissions fail validation checks due to human error. This makes SDTM automation a necessity for clinical data standardization.
Metadata-Based SDTM Automation
Many organizations today use metadata-driven approaches for SDTM automation. At the core of this method is a centralized Metadata Repository (MDR) that stores:
- Dataset specifications
- Variable definitions and mappings
- Transformation logic and rules
Using this metadata, automation frameworks apply historical mappings aligned with global standards to convert raw clinical data into SDTM format.
How SDTM is Automated with MDR
- Study teams define dataset specifications and variables in the MDR.
- The automation engine uses these specs to generate SDTM programs automatically.
- These programs transform raw clinical data into SDTM-compliant datasets—reducing manual effort and improving traceability.
Benefits of MDR-based SDTM Automation
- Consistent and traceable mappings from CRF to SDTM
- Faster build of dataset specs and transformation programs
- Centralized metadata governance by global standards teams
- Scalable, standardized data outputs across studies
However, challenges remain.
Study-specific variations, evolving standards, and inconsistencies in raw data still require manual work. Metadata alone can’t fully adapt to unexpected deviations, limiting the scalability of MDR-driven automation.
Integrating AI and machine learning with MDR frameworks can overcome these limitations. AI can detect non-standard data patterns, suggest intelligent mappings, and learn from previous transformations—automating complex, study-specific cases with greater accuracy and speed.
By combining structured metadata with adaptive AI, sponsors can move toward truly intelligent SDTM automation—scalable, consistent, and ready for real-world variability.
How AI Enhances SDTM Automation through Metadata Repositories (MDRs)
Integrating AI and machine learning into MDR-driven SDTM automation adds intelligence, adaptability, and efficiency to the process. Here’s how AI supports each stage of SDTM transformation:
1. Define the Data Model
AI helps structure a robust data model by identifying key entities, attributes, and relationships needed for SDTM mapping. This forms the foundation for automated transformation.
2. Capture Metadata
AI-powered systems extract metadata from various sources—CRFs, EDC tools, and historical datasets. This includes variables, domains, mappings, and transformation rules, ensuring metadata is comprehensive and up to date.
3. Identify and Match Source Data
One of the biggest challenges in SDTM conversion is inconsistent labeling of similar data points. For example, “Date of Birth” vs. “Birth Date.”
Using Natural Language Processing (NLP), AI understands the context and semantics of variable names and values to correctly match equivalent data across sources—eliminating ambiguity and improving mapping accuracy.
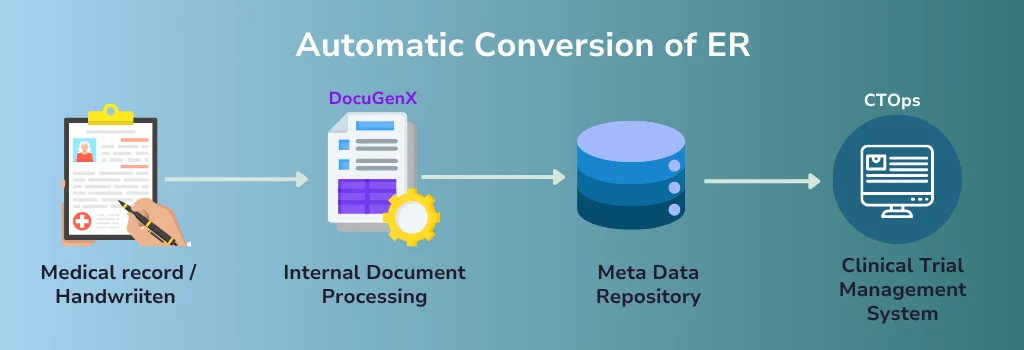
4. Define and Apply Transformation Logic
AI and ML models assist in creating SDTM-compliant transformation rules. These rules govern how raw data is derived, formatted, and mapped to SDTM variables.
5. Learn from Historical Data
Machine learning algorithms are trained on past transformation patterns. When applied to new studies, they automatically suggest or generate mappings and transformation rules based on similar variable structures—accelerating setup and reducing manual input.
From SDTM to Smart Insights: How AI Powers Clinical Understanding
Once clinical data is converted into SDTM format, it opens the door to advanced analytics. With AI layered on top, teams can move from raw data to real-time insights—enhancing safety monitoring, assessing treatment efficacy, and improving patient stratification. Here’s a look at how AI helps unlock key insights from standardized trial data:
Adverse Event Rate by Treatment Group
AI tools can automatically compare the rate and severity of adverse events—such as mild, moderate, or severe—across different treatment groups (e.g., High Dose, Low Dose, Placebo). This enables early identification of safety signals. If a specific dose group shows a spike in moderate or severe events, teams can take proactive steps to mitigate risk or adjust the study design.
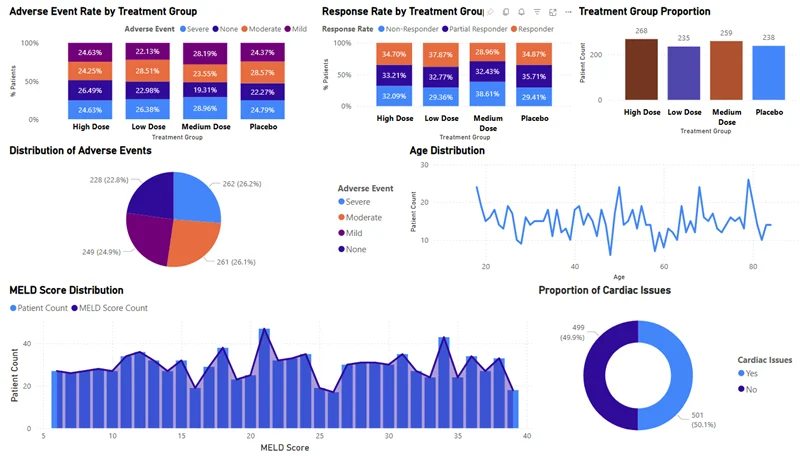
Response Rate by Treatment Group
Evaluating how many patients responded fully, partially, or not at all is critical to understanding a treatment’s effectiveness. AI can parse SDTM efficacy data to determine response rates for each treatment group. A high non-responder rate, for instance, could signal poor drug efficacy or identify subgroups that might need alternate interventions.
Treatment Group Proportion
AI can check if patients are evenly distributed across treatment arms. An unbalanced group assignment can introduce bias and compromise trial validity. With automated validation of treatment group proportions, AI ensures statistical integrity and helps maintain regulatory compliance.
Distribution of Adverse Events
Beyond treatment groups, AI can visualize the overall landscape of adverse events across the study. Whether mild symptoms are widespread or severe ones are isolated, understanding the complete picture of side effects helps sponsors assess risk profiles and adjust protocols accordingly.
Age Distribution
Age can significantly affect how patients respond to treatment. AI quickly generates insights into age demographics, ensuring representation is balanced. This supports both generalizability of the study findings and early identification of age-related treatment effects.
MELD Score Distribution
For trials involving liver disease, the MELD score (Model for End-stage Liver Disease) is a key indicator. AI tools can analyze MELD score distributions to assess baseline liver function across participants. This information guides subgroup analysis and may help predict outcomes more accurately.
Proportion of Cardiac Issues
AI algorithms can identify the percentage of patients with pre-existing cardiac conditions. For drugs with potential cardiovascular side effects, this insight is vital. If half the population has cardiac risk factors, this must be considered when interpreting safety data or making dose adjustments.
Liver Function Status
By categorizing patients based on liver function—Normal, Elevated, or Critical—AI helps clinical teams monitor hepatotoxicity risks. It also enables personalized follow-ups and helps determine whether liver function impacts treatment efficacy or adverse events.
Patient Count by Encephalopathy
Encephalopathy, a brain dysfunction often linked to liver disease, is another condition where AI provides critical insights. AI can identify prevalence in the trial population, which helps in risk stratification, protocol design, and ensuring appropriate monitoring for affected patients.
Kidney Function Status
AI breaks down renal function across the study population, categorizing patients into Normal, Elevated, or Critical ranges. These insights inform dosing strategies and safety monitoring, particularly in drugs cleared through renal pathways.
Patient Count by Biomarker Type
Biomarkers help identify response likelihood and predict adverse reactions. AI automatically categorizes patients based on biomarker types (e.g., A, B, C), enabling more targeted treatment strategies and supporting precision medicine initiatives.
Together, these insights turn structured SDTM data into a powerful decision-making engine. With AI, sponsors and researchers gain faster visibility into safety signals, patient trends, and therapeutic outcomes—ultimately accelerating the path from clinical research to real-world impact.
Key Considerations for Using AI in SDTM Automation
While AI has the potential to transform SDTM automation—making it faster, more accurate, and scalable—it’s not without its challenges. Implementing AI in a regulated and data-sensitive domain like clinical trials requires careful planning and oversight. Here are some key considerations:
1. Quality and Reliability of Input Data
AI models are only as good as the data they’re trained on. In the context of SDTM automation, this means ensuring that raw clinical data is clean, complete, and standardized before feeding it into AI/ML systems. Inconsistent variable naming, missing metadata, or non-standard formats can reduce model performance or lead to incorrect mappings. Robust pre-processing pipelines are essential to ensure the reliability of the output.
2. Importance of Domain Expertise
Clinical data is complex and context-driven. AI/ML models need to be trained on large volumes of domain-specific data to perform well—especially for tasks like mapping source variables to SDTM domains. However, acquiring this kind of labeled, contextual data can be challenging, especially while adhering to strict privacy and data protection regulations (e.g., HIPAA, GDPR). Collaborating closely with clinical data experts is key to bridging the gap between technical modeling and clinical relevance.
3. Model Interpretability and Transparency
In the pharmaceutical and life sciences industries, transparency isn’t optional—it’s a regulatory requirement. AI models used for SDTM transformation must be interpretable. Stakeholders should be able to understand how a specific mapping decision or data transformation was made. Using interpretable models or visualization tools that trace the logic behind each decision can help demonstrate compliance and build trust among regulatory teams and sponsors.
4. Continuous Learning and Regulatory Adaptation
Regulatory standards and CDISC specifications evolve over time. AI models must be able to adapt. Implementing a mechanism for periodic retraining, validation, and updating of transformation logic ensures your automation stays current. Adaptive learning strategies also help the model evolve with each study, improving performance across diverse therapeutic areas or trial designs.
Accelerating Clinical Trials with Intelligent SDTM Automation
As clinical trials grow in complexity, the need for speed, accuracy, and regulatory compliance has never been greater. Traditional SDTM conversion methods—while essential—are increasingly strained under the weight of manual effort, evolving standards, and diverse data sources.
By integrating AI and machine learning into SDTM automation, organizations can unlock new levels of efficiency, consistency, and scalability. From intelligent metadata mapping to automated domain generation, AI cuts trial time, reduces manual intervention, and intelligently adapts to study-specific variations for faster, more reliable SDTM automation.
With the right balance of technology and governance, AI-powered SDTM automation isn’t just a future-ready solution—it’s a transformative step toward smarter, more agile clinical research.


