Transforming Clinical Trials with Data-Driven Intelligence
CTOps’ Advanced Analytics module empowers stakeholders to design, monitor, and optimize clinical trials through the power of intelligent data insights. By leveraging real-world evidence, historical trial data, and predictive modeling, this solution enables more efficient study planning, enhanced operational oversight, and timely decision-making—leading to faster, more successful trials.
Unlock the Power of Advanced Analytics in Clinical Trials
Real-world data integration, predictive modeling, and unified dashboards to drive success
Real-World Data
Predictive Modeling
Risk Based Monitoring
Unified Dashbards
Unified Clinical Trials Intelligence Dashboard Suite
Global Study & Sponsor Insights
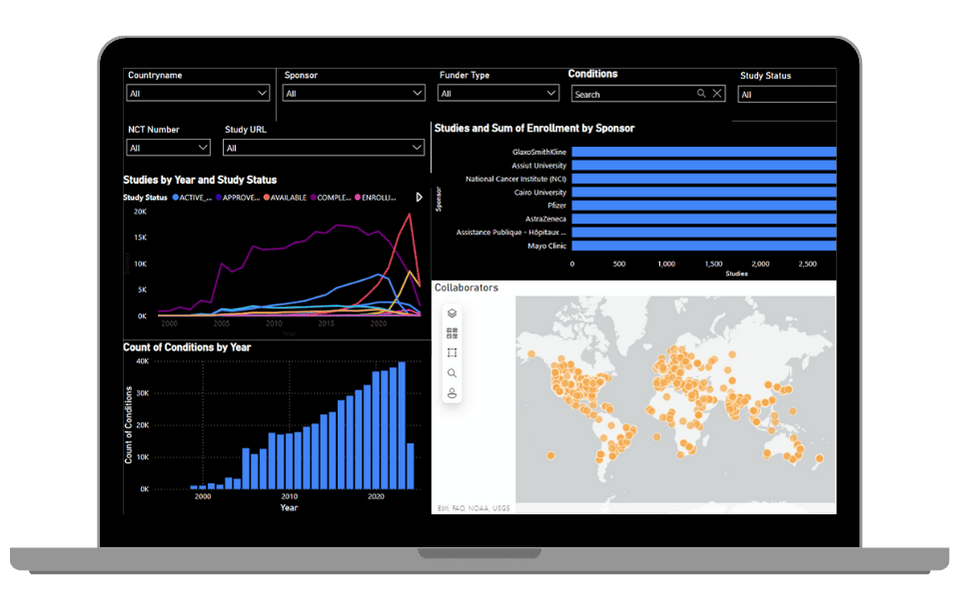
Study Analytics Dashboard
Macro-level intelligence on clinical research activity worldwide.
- Filters: Country, Sponsor, Funder Type, Condition, Study Status
- Visuals:
- Line chart: Studies by Year & Status (2000–2020)
- Bar chart: Studies & Enrollment by Sponsor
- Vertical bar chart: Conditions by Year
- Collaborator map: North America study spread
Track global trends, identify sponsor strategies, and assess geographic and therapeutic focus areas.
Disease & Trial Intelligence
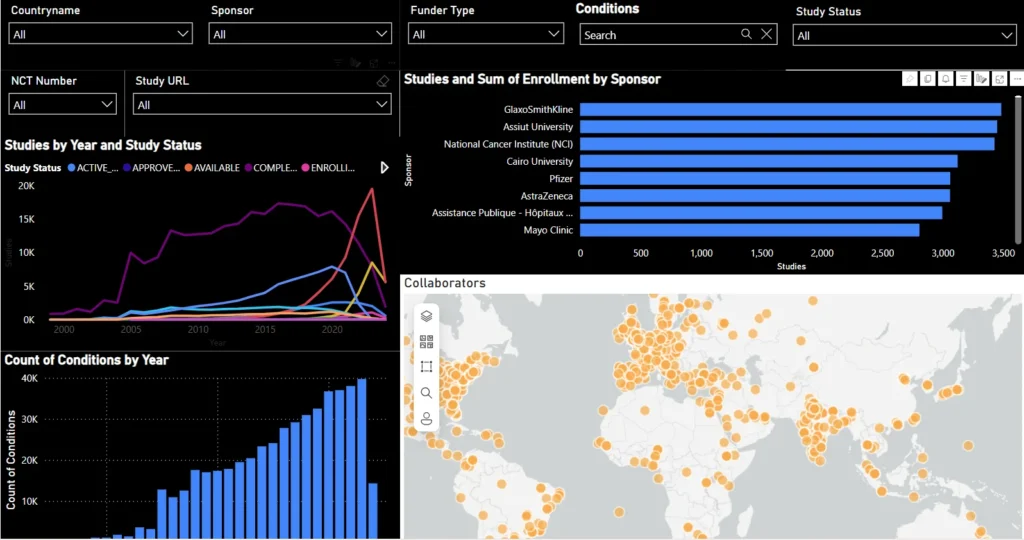
Trials Insights Dashboard
Deep dive into trials by disease area, phase, age group, and sponsor profile.
- Filters: Conditions
- Visuals:
- Bar & donut charts: Top 30 Conditions, Age Group, Study Phase, Funder Type
- Circular bar: Top Sponsors
- Table: Funder vs. Sponsor Count
Evaluate research intensity, demographic focus, and sponsor/funder behavior to support strategy and partnerships.
Site Selection & Performance Optimization
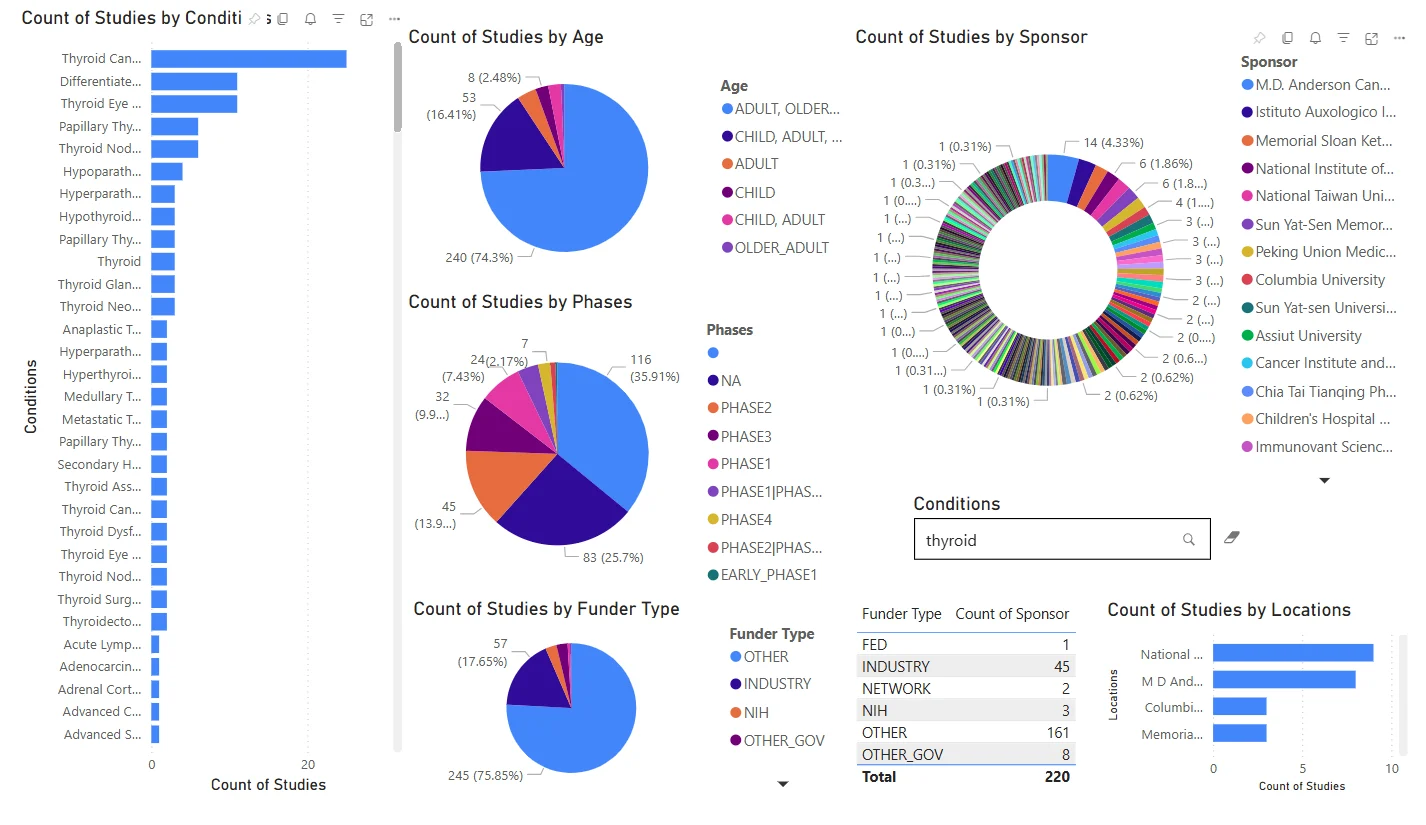
Site Performance – Site Selection Dashboard
Deep dive into trials by disease area, phase, age group, and sponsor profile.
- Filters: Conditions
- Visuals:
- Bar & donut charts: Top 30 Conditions, Age Group, Study Phase, Funder Type
- Circular bar: Top Sponsors
- Table: Funder vs. Sponsor Count
Evaluate research intensity, demographic focus, and sponsor/funder behavior to support strategy and partnerships.
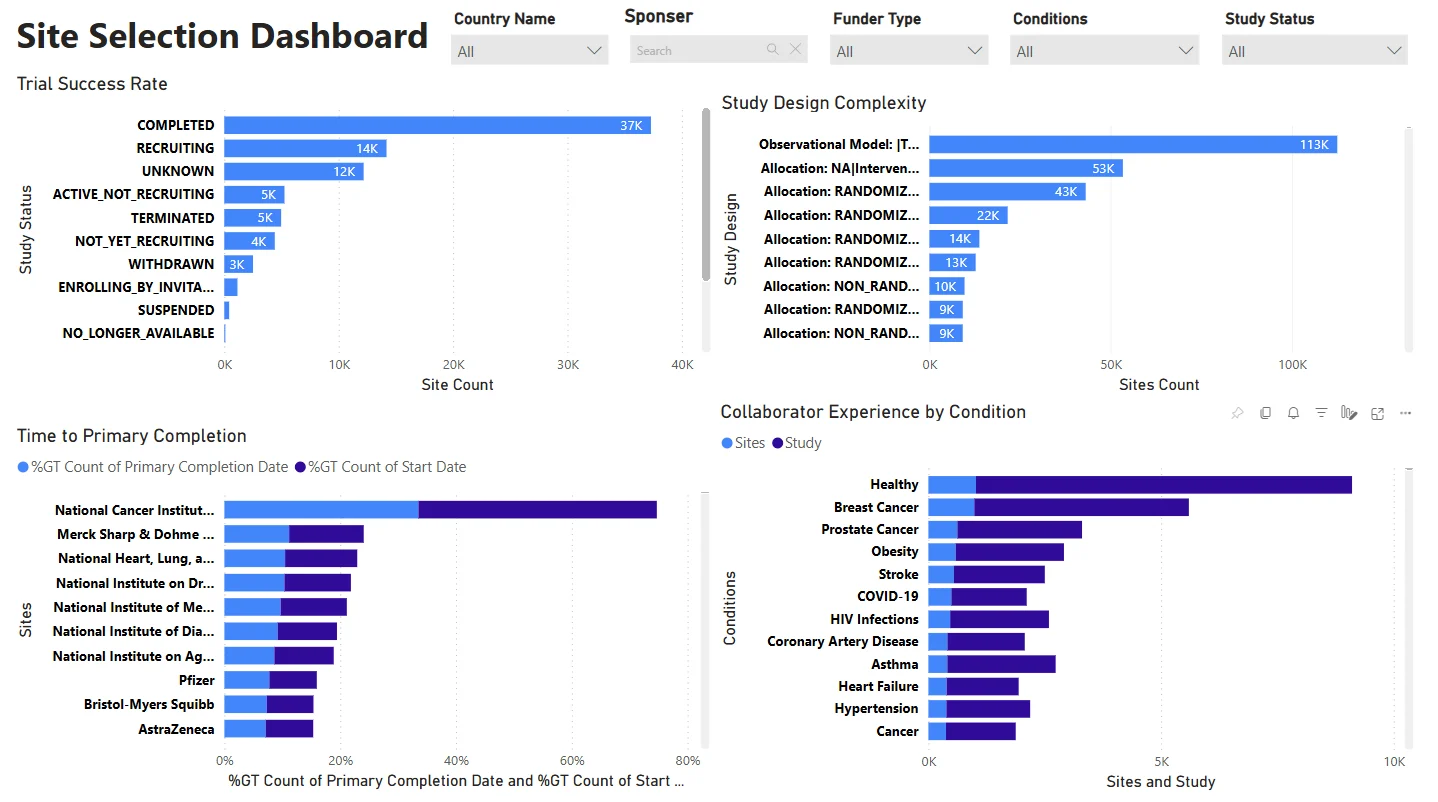
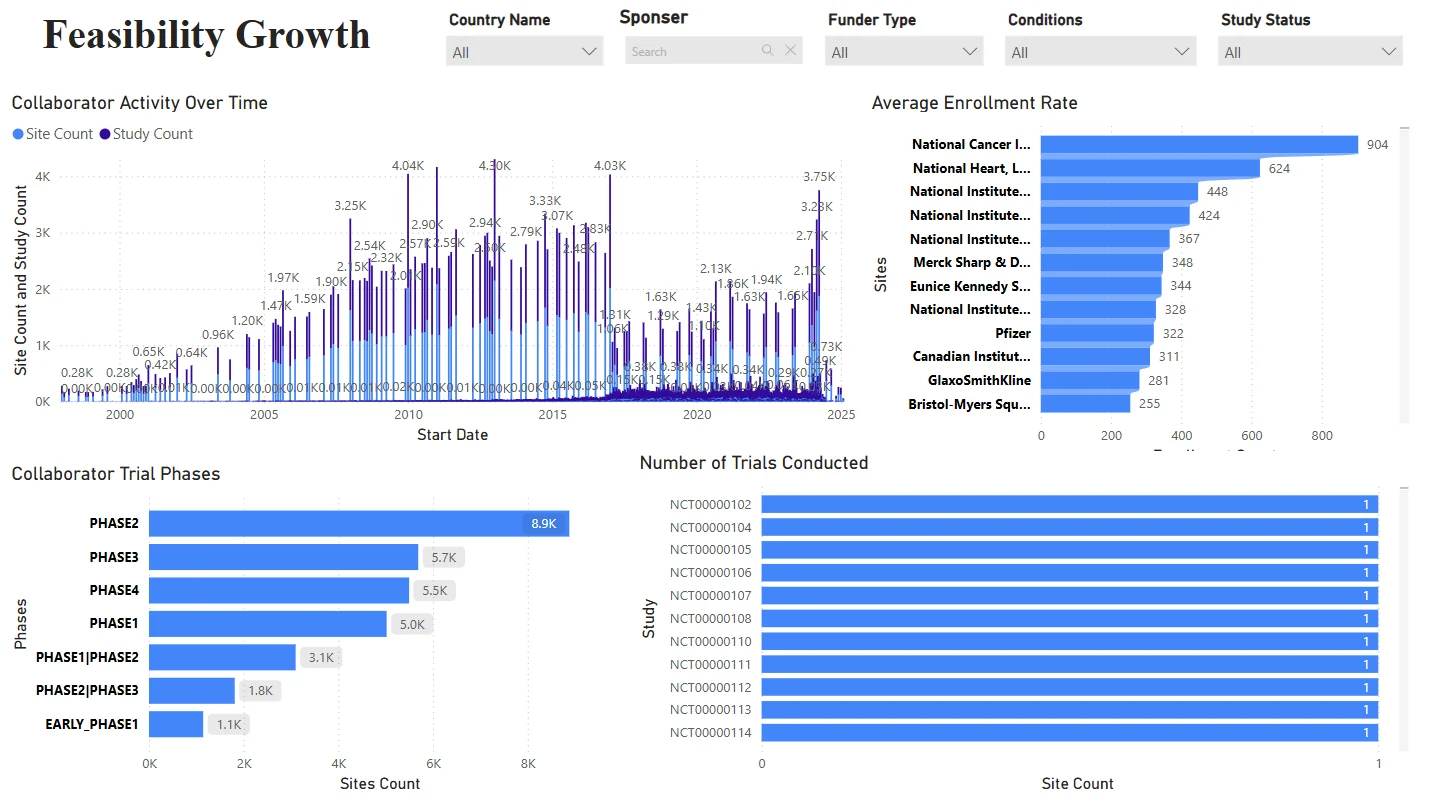
Site Performance – Feasibility Growth Dashboard
Analyze emerging site capabilities and trial history.
- Metrics: Trial Phase Experience, Enrollment Rate, Site Activity
- Export to Sheets supported
Spot “rising star” sites and optimize planning for feasibility and capacity.
RBQM & Operational Risk Dashboards
RBQM – Adverse Events Analytics
Monitor safety risks and event patterns across sites.
- Pie, line, and stacked bar charts: AEs by Site, Day, Severity
- Sankey diagram: Monthly AE flow
Enable proactive safety oversight and early intervention at risk-prone sites.

SDTM Analytics
Unlock the power of clean, compliant, and insightful clinical trial data with Mushroom Solutions’ AI-driven SDTM Analytics.
Real Impact - Oncology Trial Recuitment
Recruitment timeline improved by 28%, dropout rates reduced by 19%, saving $3.2M with a predictive, transparent data approach.
