SDTM Analytics
SDTM Analytics – Advanced Clinical Trial Data Insights
Unlock the power of clean, compliant, and insightful clinical trial data with Mushroom Solutions’ AI-driven SDTM Analytics.
Clinical trial data is the backbone of drug development. But before it drives approvals, funding, or patient access—it must be accurate, standardized, and actionable. Our SDTM Analytics solution automates SDTM validation, quality checks, and real-time reporting, enabling your trial teams to make informed decisions faster and with confidence.
Solution Features
Automated SDTM Validation
AI-powered quality checks detect missing values, formatting issues, and anomalies in real-time, ensuring submission-ready datasets.
Interactive Dashboard
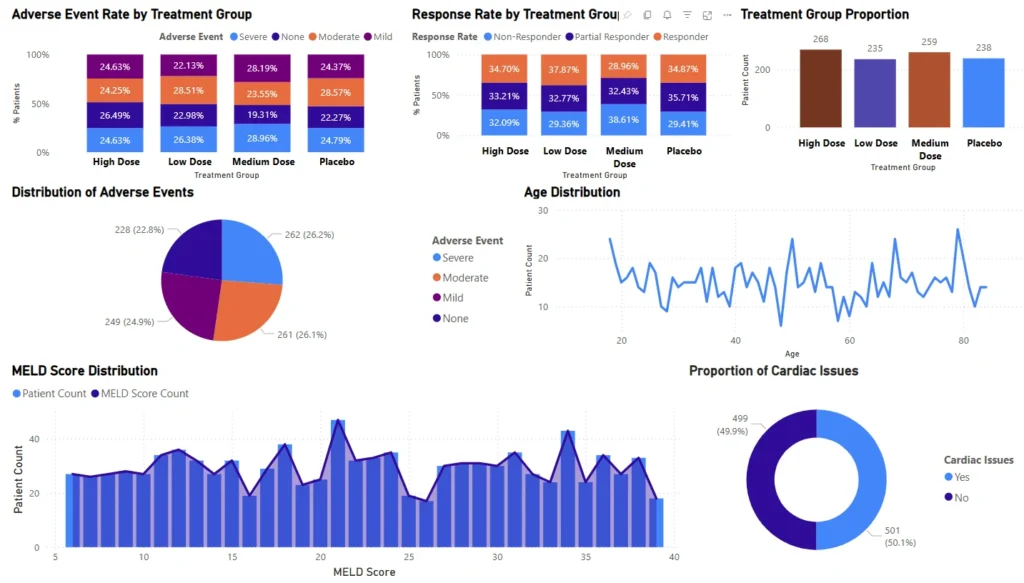
Dynamic visuals reveal critical safety and efficacy metrics across patient subgroups, enabling drill-down analysis with custom filters.
Real-Time Anomaly Detection
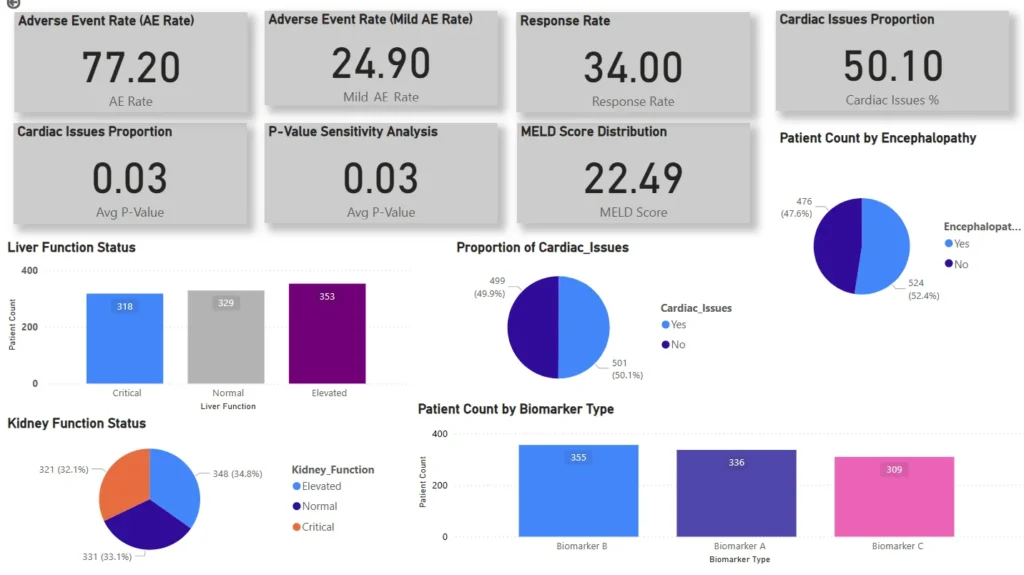
Automatically highlight high-risk scenarios such as severe adverse events or compromised liver function status.
Comprehensive Metrics
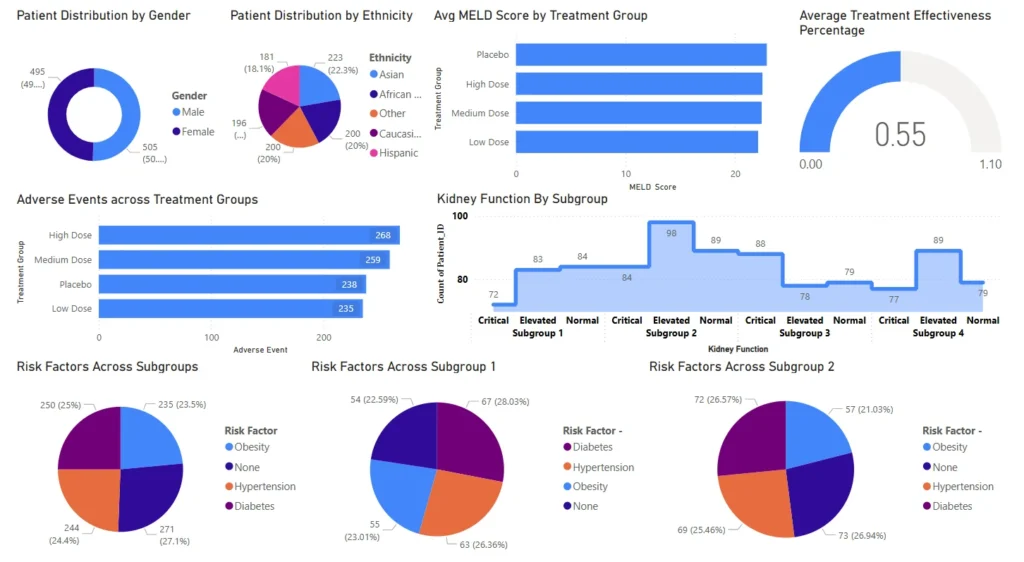
Track adverse event rates, treatment response, cardiac issues, MELD scores, kidney function, and demographic distributions.
Statistical Significance Analysis
Built-in p-value sensitivity testing confirms the reliability of observed clinical differences.
Subgroup Stratification
Analyze data by demographic, treatment group, or risk category to support targeted clinical strategies and diversity goals.
Key Benefits

Please feel free to reach out to Us
Transform your study planning with AI-powered insights and custom templates. Contact us to schedule a demo and see Smart Study Design in action!
